GetStudySolution
Getstudysolution is an online educational platform that allows students to access quality educational services and study materials at no cost.
NCERT Solutions for Class 9 Science chapter 2 – Is Matter Around Us Pure
Back Exercise
Question 1.
Which separation techniques will you apply for the separation of the following ?
- Sodium chloride from its solution in water. (CBSE 2012)
- Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
- Small pieces of metal in the engine oil of a car. (CBSE 2012, 2013, 2014)
- Different pigments from an extract of flower petals.
- Butter from curd.
- Oil from water. (CBSE 2012, 2013)
- Tea leaves from tea.
- Iron pins from sand.
- Wheat grains from husk. (CBSE 2012)
- Fine mud particles suspended in water. (CBSE 2012, 2013)
Answer:
- Evaporation : Water will evaporate leaving behind sodium chloride.
- Sublimation : Ammonium chloride will be collected as sublimate.
- Filtration : Pieces of metal can be separated by filtration.
- Chromatography : Pigments (coloured components) from the extract of flower plants can be separated by chromatography.
- Centrifugation : Butter will get separated upon centrifugation.
- Separating funnel : Oil and water can be separated by the use of separating funnel.
- Filtration : Upon filtration through a sieve, tea leaves will be collected on the sieve.
- Magnetic separation : A magnet will attract iron pins and not sand particles.
- Sieving : Wheat grains from husk can be separated with the help of sieve.
- Sedimentation : As a result of sedimentation, mud particles will settle as precipitate. It can be separated later on by filtration.
Question 2.
Write the steps you would use for making tea. Use the words-solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:
Tea can be prepared in the steps given ahead :
- Take approximately two to three cups of water (solvent) in a pan and heat it on a gas burner.
- When water starts boiling, add desired amount of milk and sugar (both are solutes).
- Now, stir with a spoon. As a result, sugar will dissolve and milk will become miscible with water. A solution will be formed.
- Further boil the solution for sometime so that sugar may completely dissolve.
- Now add the required amount of tea leaves (solute) to the pan. Boil again and filter through a sieve. Tea will be collected as filtrate. Tea leaves will get collected on sieve as residue.
Question 3.
Pragya tested the solubility of four different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
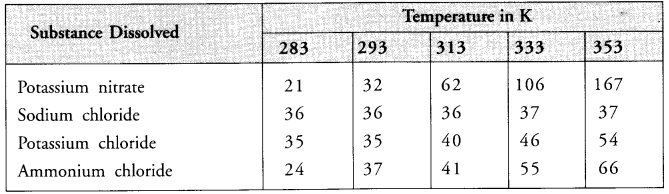
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 30 grams of water at 313 K ?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools ? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the maximum solubility at this temperature ?
(d) What is the effect of change of temperature on the solubility of a salt ?
Answer:
(a) At-313 K, in the saturated solution
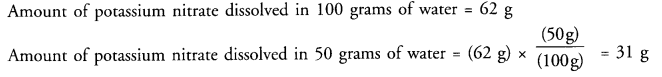
(b) When the saturated solution prepared at 353 K is cooled to room temperature (about 298 K) the solubility of potassium chloride in water will decrease. It will slowly start separating as a crystalline white residue at the bottom of the container.
(c) The solubility of the salt in a water is defined as :
the maximum amount of the salt which is dissolved in 100 g of water (or any other solvent) to form a saturated solution at a given temperature
In the light of this, at 293 K

(d) With rise in temperature, the solubility of all the salts in water increases. This has been shown by the data given in the table. Similarly, when the temperature is decreased, the solubility of these salts in water decreases.
Question 4.
Explain the following, giving examples :
(a) Saturated solution
(b) Pure substance
(c) Colloid
(d) Suspension.
Answer:
(a) Saturated solution: A solution becomes saturated if the solute starts separating at the bottom of the container in which the solution is being prepared at a given temperature. A saturated solution generally becomes unsaturated upon heating.
(b) Pure substance: A pure substance means a single substance (or matter) which cannot be separated into other kinds of matter by any physical process.
(c) Colloid: Colloidal solutions are also heterogeneous in nature like suspensions, but they have smaller size of the particles which are distributed. It ranges between 1 nm to 100 nm i.e., in between the particle size of true solution and suspension.
(d) Suspension: A suspension may be defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. They settle as precipitate if the suspension is left undisturbed for sometime.
Question 5.
Classify each of the following as a homogeneous or heterogeneous mixture :
(a) Soda water
(b) Wood
(c) Air
(d) Soil
(e) Vinegar
(f) Filtered tea.
Answer:
Homogeneous mixture : Soda water, air, vinegar, filtered tea.
Heterogeneous mixture : Wood, soil.
Air is a homogeneous mixture of different gases. However, if some dust or other particles are present, then air becomes a heterogeneous mixture.
Question 6.
How would you confirm that the colourless liquid given to you is pure water ?
Answer:
This can be confirmed by the following experiments :
- Filter the colourless liquid through a very fine filter paper. If no residue is left on the filter paper, this means that the liquid is pure water and has no suspended inpurities present in it.
- Evaporate the colourless liquid in a china dish or beaker. In case no residue is left, this means that it is pure water and has no dissolved impurities present in it.
- Determine the boiling point of pure liquid. If it comes out to be nearly 373 K (100°C), this means that the pure liquid is water.
Question 7.
Which of the following materials fall in the category of pure substances ?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air.
Answer:
Pure substances in the given list of materials are :
(a) Ice (compound)
(c) Iron (element)
(e) Calcium oxide (compound)
(f) Mercury (element).
Both milk and hydrochloric acid are homogeneous mixtures. Please note that the acid is formed when the vapours of hydrogen chloride gas are passed through water.
Wood and air (containing suspended particles) are heterogeneous mixtures. However, air free from any suspended particles is a homogeneous mixture.
Question 8.
Identify the solutions among the following mixtures :
(a) Soil
(b) Sea water
(c) Air
(d) Coal
(e) Soda water.
Answer:
By definition, a solution or homogeneous mixture is the mixture of two or more non-reacting substances present in a single phase. In the light of this, the solutions among the following are :
(b) Sea water
(c) Air
(e) Soda water.
Question 9.
Which of the following will show “Tyndall effect” ?
- Salt solution
- Milk
- Copper sulphate solution
- Starch sol.
Answer:
Tyndall effect is shown by colloidal sol. Since milk and starch sol are colloidal sol therefore, these will show Tyndall effect.
Question 10.
Classify the following into elements, compounds and mixtures :
- Sodium
- Soil
- Sugar solution
- Silver
- Calcium carbonate
- Tin
- Silicon
- Coal
- Air
- Soap
- Methane
- Carbon dioxide
- Blood.
Answer:
Elements : Sodium, Silver, Tin and Silicon
Compounds : Calcium carbonate, Methane, Carbon dioxide.
Mixtures : Soil, Sugar solution, Coal (as percentage of carbon varies), Air, Blood, Soap.
Question 11.
Which of the following are chemical changes ?
- Growth of a plant
- Rusting of iron
- Mixing of iron filings and sand
- Cooking of food
- Digestion of food
- Freezing of water
- Burning of a candle.
Answer:
Chemical changes are : Growth of a plant, Rusting of iron, Cooking of food, Digestion of food, Burning of a candle.
In-Text Questions
Question 1.
What is meant by a pure substance ?
Answer:
Substances which are made up of only one kind of atoms or molecules and have a definite composition throughout are known as pure substances.
Question 2.
List points of differences between homogeneous and heterogeneous mixtures.
Answer:
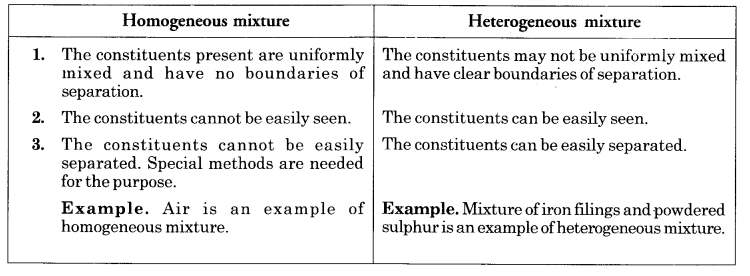
Question 3.
How are sol, solutions and suspension different from each other ?
Answer:
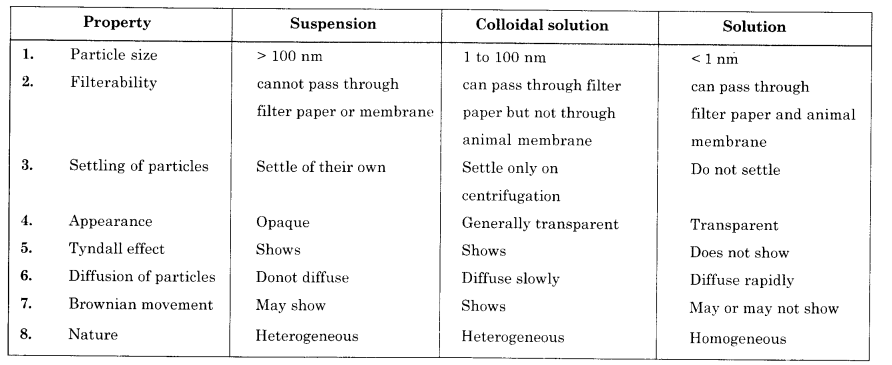
Question 4.
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:

Question 5.
How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C) which are miscible with each other ?
Answer:
The separation can be done by applying process of simple distillation. Both are miscible liquids. Since the difference in the boiling points is less than 25 K, the separation can be done with the help of fractional distillation technique. Petrol with less boiling point distils first leaving behind kerosene in the distillation from flask.
Question 6.
Name the technique to separate
- butter from curd
- salt from sea water
- camphor from salt.
Answer:
- The separation can be done by carrying centrifugation in a centrifugal machine.
- The separation can be done either by crystallisation or by evaporation.
- The process of sublimation helps in separating camphor from salt. Camphor undergoes sublimation.
Question 7.
What types of mixtures can be separated by technique known as crystallisation ?
Answer:
The solid mixtures in which one component or impurity is less soluble in a particular solvent as compared to the other. For example, impure samples of copper sulphate, potassium nitrate, potash alum etc. can be purified by this method.
Question 8.
Classify the following into physical and chemical changes.
- Cutting of trees
- Melting of butter in a pan
- Rusting of almirah
- Boiling of water to form steam
- Passing of electric current through water and the water breaking down into hydrogen and oxygen gases
- Dissolving common salt in water
- Making of fruit salads with raw fruits
- Burning of paper and wood.
Answer:
Please note that a change which can be easily reversed is a physical change while the one which cannot be reversed is a chemical change in nature. Based on this concept, the changes that are listed may be classified as :
- Chemical change
- Physical change
- Chemical change
- Physical change
- Chemical change
- Physical change
- Physical change
- Chemical change.
Question 9.
Try to segregate the things around you as pure substances and mixtures :
(a) distilled water
(b) curd
(c) diamond
(d) ice cream
(e) kerosene
(f) cooking oil
(g) steel
(h) graphite
(i) raw rubber
(j) vulcanised rubber
(k) solder wire.
Answer:
Pure substances : Distilled water, diamond, graphite, raw rubber
Mixtures : curd, ice cream, kerosene oil, cooking oil, steel, vulcanised rubber, solder wire (alloy of lead and tin)