GetStudySolution
Getstudysolution is an online educational platform that allows students to access quality educational services and study materials at no cost.
NCERT Solutions for Class 8 Science chapter 6 – combustion and Flame
Back Exercise
Question 1:
List conditions under which combustion can take place.
Answers
The burning of a substance in the presence of oxygen is defined as combustion.
The conditions under which combustion can take place are:
⚬ The presence of air or oxygen.
⚬ The presence of fuel plays a significant role.
⚬ Ignition temperature is maintained (It is defined as the substance that catches fire at its lowest temperature.)
Question 2:
Fill in the blanks.
(a) Burning of wood and coal causes __________ of air.
(b) A liquid fuel, used in homes is __________.
(c) Fuel must be heated to its before it __________ starts
burning.
(d) Fire produced by oil cannot be controlled by __________.
Answers
(a) pollution
(b) kerosene
(c) ignition temperature
(d) water
Question 3:
Explain how the use of CNG in automobiles has reduced pollution in
our cities.
Combustion of fuels like petroleum causes formation of un-burnt carbon particles along with carbon monoxide gas. These harmful pollutants enter the air and cause respiratory diseases. Compressed Natural Gas (CNG) produces these harmful products in very less quantity. It is a comparatively cleaner fuel. Therefore, the use of CNG has reduced pollution in our cities.
Question 4:
Compare LPG and wood as fuels.
Answers
Wood
⚬ It is considered as a traditional fuel used for both domestic and industrial purposes.
⚬ Wood produces a lot of smoke which pollutes the atmosphere and cause respiratory diseases.
⚬ The usage of wood to a large extent causes deforestation.
⚬ The calorific value of wood ranges between 17000 to 22000 kJ/kg
⚬ However, wood may be used as a furnace, stove or fireplace in indoors while it is used for a campfire, furnace at outdoors.
LPG
⚬ The usage LPG (Liquefied petroleum gas) has replaced wood.
⚬ It doesn’t release smoke and other pollutants
⚬ It is a cleaner fuel
⚬ The fuel efficiency of LPG is more than that of wood.
⚬ The calorific value of LPG is 55000 kJ/kg
⚬ Hence LPG is mostly preferred choice
Question 5:
Give reasons.
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped
around an aluminium pipe does not.
Answers
(a) Water is a conductor of electricity, so it can easily conduct electric current and cause danger of electric shocks or short-circuits. Therefore, water can not be used to control the fire involving electrical equipment.
(b) LPG is a better domestic feul as it is readly available and is cheap. it burns easly in air at a moderate rate andit produces a large amount of heat. It does not produces smoke and un-burnt carbon particles, which cause respiratory problems.
(c) Paper by itself catches fire easily because it has low ignition temperature but when wrapped around an aluminium pipe its temperature is lowered due to aluminium metal absorbing the heat supplied to paper. So it does not catch fire.
Question 6:
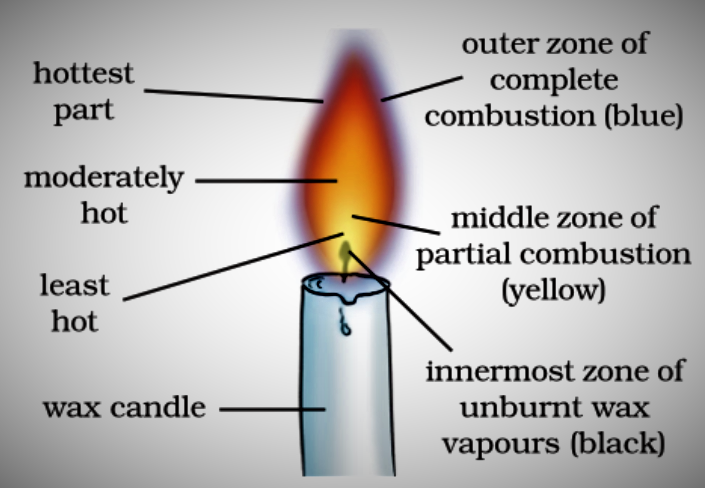
Make a labelled diagram of a candle flame.
Answers
Question 7:
Name the unit in which the calorific value of a fuel is expressed.
Answers
Calorific value is defined as the amount of energy contained in the fuel. It is expressed as kilojoules per kilogram (kJ/kg), where kJ=kilo joules and kg=kilogram.
Question 8:
Explain how CO2 is able to control fires.
Answers
CO2 is a non-combustible gas and it is also non supporter of combustion. It extinguishes fire in two ways:
(i) Since it is heavier than oxygen, it covers the fire like a blanket and cuts off the contact between oxygen and fuel.
(ii) In cylinders, CO2 is kept in the liquid form. When released, it expands enormously and cools down. This brings down the temperature of the fuel, which helps in controlling the fire.
Question 9:
It is difficult to burn a heap of green leaves but dry leaves catch fire easily.
Explain.
Answers
A heap of green leaves contains a lot of moisture in it, hence its ignition temperature is high. Therefore it does not catch fire easily. But dry leaves have no moisture content in it, hence its ignition temperature is low. Therefore it catches fire easily.
Question 10:
Which zone of a flame does a goldsmith use for melting gold and silver
and why?
Answers
The goldsmith mainly uses non-luminous flame which is termed to be the outermost part of the flame. This part of the flame is used because the outermost flame undergoes complete combustion and is considered as the hottest part of the flame.
Question 11:
In an experiment 4.5 kg of a fuel was completely burnt. The heat produced
was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Answers
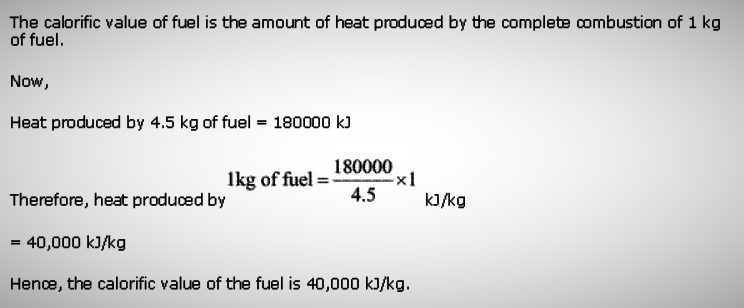
Question 12:
Can the process of rusting be called combustion? Discuss.
Answers
The process of rusting cannot be considered to be an example of combustion, as combustion involves the intanteous oxidation of a substance. Rather, rusting of iron is considered to be a process of slow oxidation, where the uppermost iron layer gets degenerated due to reaction with oxygen, and the gain in weight is also observed.
Question 13:
Abida and Ramesh were doing an experiment in which water was to be
heated in a beaker. Abida kept the beaker near the wick in the yellow part
of the candle flame. Ramesh kept the beaker in the outermost part of the
flame. Whose water will get heated in a shorter time?
Answers
The water placed in the outermost part of the flame will be heated in a short time since it is non-luminous flame and is regarded as the hottest part of the flame. So Ramesh’s beaker will be heated first. However, Abida who placed the beaker in the luminous flame (yellow flame)is comparatively less hot.