GetStudySolution
Getstudysolution is an online educational platform that allows students to access quality educational services and study materials at no cost.
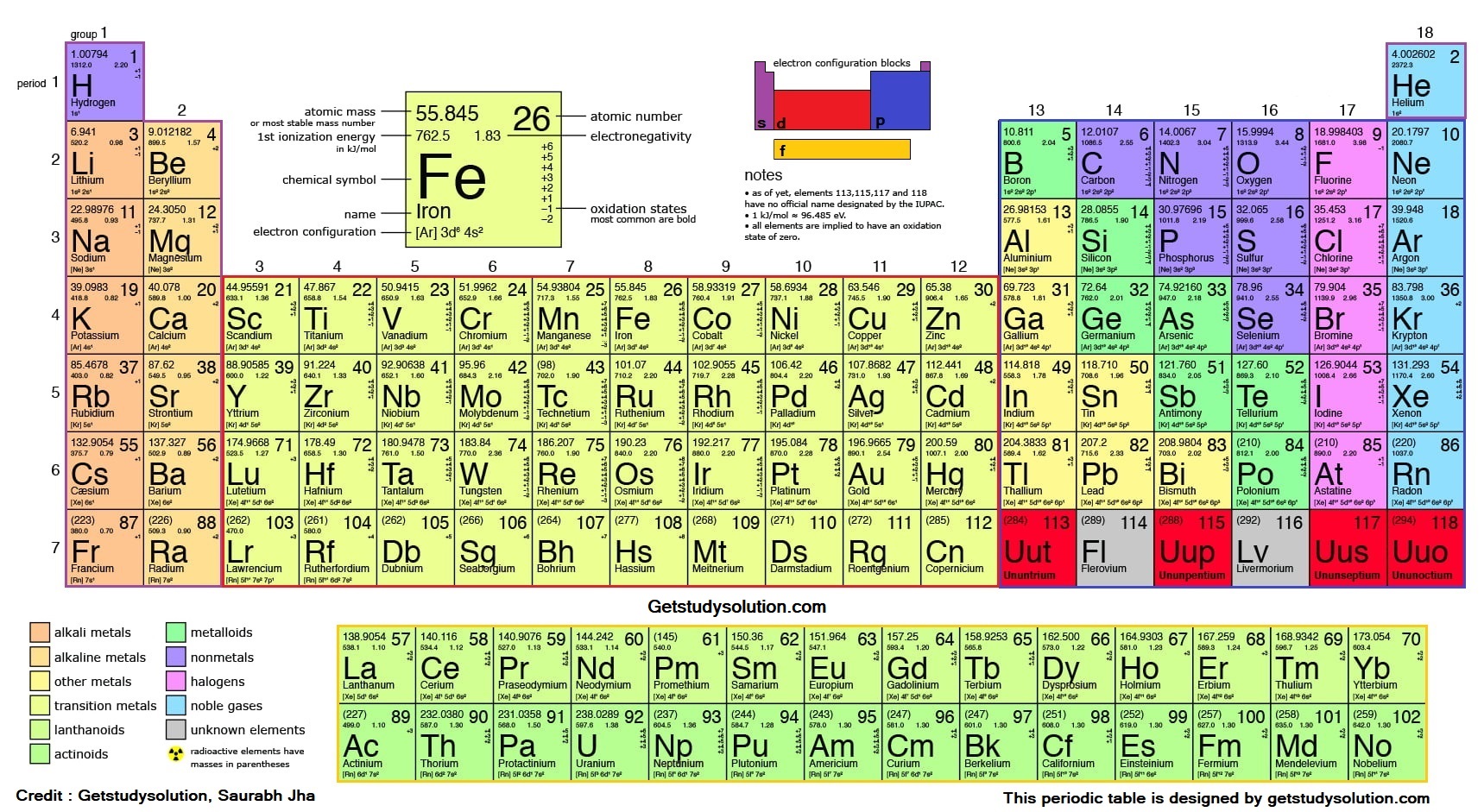
History - Mendeléev's contribution
The main credit for classifying elements goes to Dmitri Ivanovich
Mendeléev, a Russian chemist. He was the most important contributor
to the early development of a Periodic Table of elements wherein the
elements were arranged on the basis of their fundamental property, the
atomic mass, and also on the similarity of chemical properties.
In 1869, when Mendeléev started his work, 63 elements were known. He
examined the relationship between the atomic masses of the elements
and their physical and chemical properties. Among chemical properties,
Mendeléev concentrated on the compounds formed by elements with
oxygen and hydrogen. He selected hydrogen and oxygen as they are
very reactive and formed compounds with most elements. The formulae
of the hydrides and oxides formed by an element were treated as one of
the basic properties of an element for its classification. He then took 63
cards and on each card he wrote down the properties of one element. He
sorted out the elements with similar properties and pinned the cards
together on a wall. He observed that most of the elements got a place in
a Periodic Table and were arranged in the order of their increasing atomic
masses. It was also observed that there occurs a periodic recurrence of
elements with similar physical and chemical properties. On this basis,
Mendeléev formulated a Periodic Law, which states that ‘the properties
of elements are the periodic function of their atomic masses’>
Mendeléev’s Periodic Table contains vertical columns called ‘groups’
and horizontal rows called ‘periods’

In 1913, Henry Moseley showed that the atomic number (symbolised as Z) of an element is a more fundamental property than its atomic mass. Accordingly, Mendeléev’s Periodic Law was modified and atomic number was adopted as the basis of Modern Periodic Table and the Modern Periodic Law can be stated as follows:
‘Properties of elements are a periodic function of their atomic number.’
Trends in periodic table
Periodic trends are those which explain certain properties of elements that are present in the periodic table. The periodic trends are electronegativity, ionic radius, atomic radius, electron affinity, ionization energy, chemical reactions of elements, and metallic character.
Period and Group:
- The elements which are arranged horizontally (from left to right) are called periods.
- The elements which are arranged vertically (from top to bottom) are called a group.
Factors Influencing Periodic Trends:
The total number of protons present in the nucleus, the total number of energy levels and the shielding effects are the major factors that influence the periodic trends.
Electronegativity:
The property of an atom to attract a pair of electrons is known as Electronegativity. The size of an atom and nuclear charge affects the electronegativity of the elements. It increases as we move from left to right of the periodic table and decreases as we move from top to bottom of the periodic table.
Fluorine has higher electronegative whereas caesium has less electronegative value. It also varies among metals and non - metals. Non - metals are more electronegative than metals. It also helps to identify the types of bonds formed between the elements.
Ionic radius:
Ionic radius is defined as the radius of the ions of the elements that are present in the periodic table. Ions are formed either by gaining or losing electrons of an atom.
The distance between the center of the nucleus and the maximum end of an ion is known as ionic radius. Anions have a higher ionic radius than cations. When the atomic number is increased, the ionic radius also gets increased. Potassium ions have a higher ionic radius than lithium ions because of the increase in atomic number.
Atomic radius:
Atomic radius is defined as the size of an atom, and it is determined by measuring the distance between the nucleus and the outermost electron of an atom.
It decreases as we move from left to right of the periodic table and increases as we move from top to bottom. Covalent radius(for non - metals) and metallic radius (for metals) can be determined by the concept of atomic radius. It also depends on the number of protons and the attraction between the electrons and the protons.
Electron affinity:
The addition of electrons to a neutral gaseous atom releases a certain amount of energy. This is known as electron affinity. A negative ion or anion is formed as a result of electron affinity. It increases from left to right and decreases from top to bottom of the periodic table. As the atomic size increases, electron affinity decreases and vice - versa. The screening effect and reactivity of non - metals also affect the electron affinity.
Ionization energy:
Ionization energy is defined as the maximum energy required to remove an electron from an atom, as it is difficult to remove. The nature of the chemical bonds and the geometry of the molecule depends on this ionization energy. It increases from left to right and decreases from top to bottom of the periodic table.
Metallic character:
Metallic character is defined as the characters that are associated with the metals which are present on the periodic table. These characters include metallic luster, hardness, malleability, thermal conductivity, etc.,
The elements which are present on the left side of the periodic table have a higher metallic character. It decreases from left to right because of the addition of electrons and increases from top to bottom because of the removal of electrons. When the atomic number is higher, the ability to lose electrons is also higher.
Melting point:
The total energy required to change the solid substance into a liquid is known as the melting point. If the bond is strong between the atoms of elements, high energy is required to break the bond.
It decreases as we move from top to bottom. But, for non - metals, it increases from top to bottom of the periodic table.
Carbon has a high melting point among metals. Boron has a high melting point among semi-metals. Tungsten has a high melting point among metals.
Non - metallic character:
Non - metallic character of elements increases from left to right and decreases from top to bottom of the periodic table. The elements which have this character do not have the metallic property.
Valency:
The electronic configuration determines the valency of elements. It varies from left to right in a row and remains the same from top to bottom in a group.
Periodic Table - More To Know
Periods
: lst Period contains only two elements namely Hydrogen (H), Helium (He). It is called as shortest period.2nd period starts with Lithium (Li) and contains eight elements.
Li, Be, B, C, N, O, F, Ne.
3rd period starts with Sodium (Na) and contains eight elements.
Na, Mg, Al, Si, P, S, Cl, Ar.
Note : 2nd and 3rd periods are called as short-periods.
4th period contains eighteen elements starting with Potassium (K).
K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr.
5th period contains eighteen elements starting from Rubiditim (Rb).
Rb,.........., Xe(Xenon).
Note : IV and Vperiods are called as long-periods.
6th period consists of 32 elements, starting from Cesium (Cs) and ending with Radon (Rn). It is called as longest period. Apart from the representntive and transition elements this period also contains Inner transition elements called as Lanthanides. (Ce,......, Lu).
7th period is incomplete period and at present contains 19 elements starting form Francium (Fr). upto Uranium (U) all the elements are naturally occurring but rest are radioactive with very short halt-lives. These also include a part ot inner transition elements, called as actinides (Th,......, Lr).
Groups
: Group 1 consists of H (1sˡ), Li (2sˡ), Na(3sˡ),... The common outermost electronic configuration is ns1. Elements belonging to this group are called as Alkali Metals.Group 2 consists of Be (2s²), Mg (3s²), Ca (4s²),.... The common electronic configuration is ns2. Elements of this group are called as Alkaline Earth Metals.
Group 13 consists of B (2s² 2pˡ), Al (3s² 3pˡ),. The common electronic configuration is ns² npˡ. Elements of this group are called as Boron Family.
Grou p 14 contains C (2s² 2p²), Si (3s² 3p²),... The common electronic configuration is ns² np². This group is known as Carbon Family.
Group 15 contains N (2s² 2p³), P (3s² 3p³),... The common electronic configuration is ns²np³. This group is known as Nitrogen Family. The elements of this group are also called as PNICTOGENS (poisonous compounds forming elements).
Group 16 contains O (2s² 2p⁴), S (3s² 3p⁴),.... The common electronic configuration is ns² np⁴. This group is known as Oxygen Family. The elcments of this group are also famous as CHALCOGENS (ore-forming elements).
Group 17 contains F (2s² 2p⁵), Cl (3s² 3p⁵),.... The common electronic configuration is ns² np⁵. Elements of this group are called as HALOGENS (salt forming elements).
Group 18 (or Zero group) contains He (1s²), Ne (2s² 2p⁶), Ar (3s² 3p⁶),..... The common electronic configuration is ns² np⁶. Elements of this group are called as Inert Gases or Nobles Gases
Features of Groups 1, 2, 13 - 18
Elements belonging to these groups are in general called as Representative Elements.
General Electronic configuration for group 1, 2 can be written as ns² and ns² npˡ to ns² np⁶ - for group 13 - 18.
n: here represents the number of period to which a particular elements belongs (principal quantum number).
The total number of electrons i.e., number of electrons in s & p sub-shells gives the number of group to which a particular elements belongs.
Elements of 1 and 2 groups are also called as s-block elements, as final electron in these elements (also called as differentiating electron) enters s sub-shell. Elements of 13 to 18 groups are called as p-block elements, as differentiating electron in these elements enters p sub-shell.
Features of Groups 3 - 12
Groups 3 to 12 are known as transition elements or d-block elements, as the differentiating electron (last electron) in these elements enters d-sub-shell. General electronic configuration of these elements can be written as (n-1)d10 ns02
Group 3 has a special feature in sense that, it contains elements in which the differentiating electron enters the f sub-shell, hence these elements are also called as f-block elements apart from being called as Inner Transition elements. These are placed in two horizontal rows below the table and are called as Lanthanides (also called as Lanthanoides) and Actinides (also called as Actinoids).
Typical Elements
Elements of third period are also called as Typical Elements. These include Na, Mg, Al, Si, P, S, CI. The properties of all the elements belonging to a particular group resemble the properties of the corresponding typical element of that group. For example, the general properties of Alkali Metal can be predicted from the behaviour of Na, not Li, the first member of the family.
The typical elements (all having n = 3) can take up 18 electrons. Note that, for these elements 3d sub-shell is available, but it is not filled i.e., these have vacant d sub-shell. This is not the case with second period elements, hence they have somewhat different properties than the rest of the group or we can say that it is the typical element, which in true sense represents a group.
Bridge Elements
Elements of second period are also called as Bridge Elements. The properties of these elements resemble with the properties of elements belonging to third period placed diagonally.
Noble or Inert Gases
Elements of group 18 or zero group are called Inert or Noble Gases. They have completely filled (2 or 8 electrons in outermost shell) outermost shells, called as stable configuration. Their valency is zero. They are almost inert in their chemical behaviour. They have weak intermolecular forces in them and hence are gases and exist in monatomic states. Classification of Elements based on their position is the periodic table.
1. Metals
This is the largest class of the elements. This includes elements belonging to 1, 2, 3 to 12 groups (i.e., all transition and inner-transition elements) and some elements of groups 13 to 15 lying near the bottom of the table. The metals are characterized by their nature of readily giving up the electron apart from shinning lusture. The oxides of metals are basic in nature.
2. Non-metals
These do not give up electron, in fact like to take up the electron to form negative ion. These include 10 elements lying to the right side of the table. They are C, N, O,F (2nd period), P, S, CI (3rd period), Se, Br (4th period) and I (5th period). The oxides of non-metals are acidic in nature.
3. Metalloid
You can very easily observe that metallic character has decreased when one moves to the right of the table across a row. It is observed that some elements lying at the border of metallic and non-metallic behaviour, exhibit both the metallic and non-metallic character, these are called as metalloids. These include 8 elements namely: B. Si, Ge, As, Sb, Te, Po and At. The oxides of metalloids are generally amphoteric in nature.
Note: The elements in group 18 do not behave like metals, nor do they behave like non-metals. So they are classified separately as Noble Gases. Also the element Hydrogen (H) is different from any other element and cannot be easily classified into a particular group (however it is placed along with the Alkali Metals, though it does not exhibit metallic character).